What Ions Do Bases Produce
Definitions of Acids and Bases
and the Office of Water
Properties of Acids and Bases Co-ordinate to Boyle
In 1661 Robert Boyle summarized the backdrop of acids as follows.
1. Acids have a sour gustation.
2. Acids are corrosive.
3. Acids change the color of sure vegetable dyes, such as litmus, from blue to ruddy.
4. Acids lose their acidity when they are combined with alkalies.
The name "acrid" comes from the Latin acidus, which means "sour," and refers to the sharp odor and sour taste of many acids.
Examples: Vinegar tastes sour because information technology is a dilute solution of acetic acid in water. Lemon juice tastes sour because it contains citric acid. Milk turns sour when it spoils because lactic acid is formed, and the unpleasant, sour odor of rotten meat or butter can exist attributed to compounds such as butyric acid that class when fat spoils.
In 1661 Boyle summarized the properties of alkalies every bit follows.
- Alkalies experience slippery.
- Alkalies change the color of litmus from blood-red to blue.
- Alkalies become less alkaline when they are combined with acids.
In essence, Boyle defined alkalies every bit substances that consume, or neutralize, acids. Acids lose their characteristic sour taste and ability to dissolve metals when they are mixed with alkalies. Alkalies even contrary the change in color that occurs when litmus comes in contact with an acrid. Eventually alkalies became known as bases because they serve every bit the "base" for making certain salts.
![]()
The Arrhenius Definition of Acids and Bases
In 1884 Svante Arrhenius suggested that salts such as NaCl dissociate when they dissolve in h2o to give particles he chosen ions.
| H2O | ||
| NaCl(s) | | Na+(aq) + Cl-(aq) |
Three years afterward Arrhenius extended this theory by suggesting that acids are neutral compounds that ionize when they dissolve in water to give H+ ions and a corresponding negative ion. According to his theory, hydrogen chloride is an acrid considering it ionizes when information technology dissolves in water to give hydrogen (H+) and chloride (Cl-) ions equally shown in the figure below.
| HtwoO | ||
| HCl(g) | | H+(aq) + Cl-(aq) |
Arrhenius argued that bases are neutral compounds that either dissociate or ionize in water to give OH- ions and a positive ion. NaOH is an Arrhenius base of operations because information technology dissociates in h2o to give the hydroxide (OH-) and sodium (Na+) ions.
| HtwoO | ||
| NaOH(southward) | | Na+(aq) + OH-(aq) |
An Arrhenius acid is therefore any substance that ionizes when it dissolves in h2o to give the H+, or hydrogen, ion.
An Arrhenius base is any substance that gives the OH-, or hydroxide, ion when it dissolves in water.
Arrhenius acids include compounds such as HCl, HCN, and HtwoSOiv that ionize in water to give the H+ ion. Arrhenius bases include ionic compounds that contain the OH- ion, such equally NaOH, KOH, and Ca(OH)2.
This theory explains why acids have similar properties: The characteristic properties of acids result from the presence of the H+ ion generated when an acrid dissolves in water. It besides explains why acids neutralize bases and vice versa. Acids provide the H+ ion; bases provide the OH- ion; and these ions combine to form water.
H+(aq) + OH-(aq) ![]() HiiO(l)
HiiO(l)
The Arrhenius theory has several disadvantages.
- It can be practical only to reactions that occur in water considering it defines acids and bases in terms of what happens when compounds dissolve in water.
- Information technology doesn't explain why some compounds in which hydrogen has an oxidation number of +1 (such as HCl) deliquesce in water to requite acidic solutions, whereas others (such as CHiv) do non.
- Only the compounds that comprise the OH- ion can be classified as Arrhenius bases. The Arrhenius theory can't explain why other compounds (such equally Na2COthree) accept the characteristic backdrop of bases.
![]()
The Role of H + and OH - Ions In the Chemistry of Aqueous Solutions
Becuase oxygen (EN = 3.44) is much more electronegative than hydrogen (EN = 2.twenty), the electrons in the H![]() O bonds in water aren't shared equally by the hydrogen and oxygen atoms. These electrons are drawn toward the oxygen atom in the center of the molecule and away from the hydrogen atoms on either finish. As a result, the h2o molecule is polar. The oxygen cantlet carries a partial negative charge (
O bonds in water aren't shared equally by the hydrogen and oxygen atoms. These electrons are drawn toward the oxygen atom in the center of the molecule and away from the hydrogen atoms on either finish. As a result, the h2o molecule is polar. The oxygen cantlet carries a partial negative charge (![]() -), and the hydrogen atoms bear a partial positive charge (
-), and the hydrogen atoms bear a partial positive charge (![]() +).
+).

When they dissociate to form ions, h2o molecules therefore form a positively charged H+ ion and a negatively charged OH- ion.
![]()
The opposite reaction can also occur ![]() H+ ions can combine with OH- ions to form neutral water molecules.
H+ ions can combine with OH- ions to form neutral water molecules.
![]()
The fact that water molecules dissociate to form H+ and OH- ions, which can then recombine to form water molecules, is indicated by the following equation.
![]()
![]()
To What Extent Does Water Dissociate to Course Ions?
At 25C, the density of water is 0.9971 g/cm3, or 0.9971 g/mL. The concentration of h2o is therefore 55.35 molar.

The concentration of the H+ and OH- ions formed past the dissociation of neutral H2O molecules at this temperature is only 1.0 x x-7 mol/L. The ratio of the concentration of the H+ (or OH-) ion to the concentration of the neutral H2O molecules is therefore ane.8 ten ten-9.

In other words, only most 2 parts per billion (ppb) of the water molecules dissociate into ions at room temperature. The figure below shows a model of 20 water molecules, one of which has dissociated to course a pair of H+ and OH- ions. If this illustration was a very-high-resolution photograph of the construction of water, nosotros would see a pair of H+and OH- ions on the boilerplate of just once for every 25 meg such photographs.

![]()
The Operational Definition of Acids and Bases
The fact that water dissociates to form H+ and OH- ions in a reversible reaction is the ground for an operational definition of acids and bases that is more than powerful than the definitions proposed past Arrhenius. In an operational sense, an acrid is whatever substance that increases the concentration of the H+ ion when it dissolves in water. A base is whatsoever substance that increases the concentration of the OH- ion when it dissolves in water.
These definitions tie the theory of acids and bases to a uncomplicated laboratory test for acids and bases. To decide whether a compound is an acid or a base we dissolve information technology in water and exam the solution to see whether the H+ or OH- ion concentration has increased.
![]()
Typical Acids and Bases
The properties of acids and bases result from differences between the chemical science of metals and nonmetals, as tin can be seen from the chemistry of these classes of compounds: hydrogne, oxides, and hydroxides.
Compounds that comprise hydrogen jump to a nonmetal are called nonmetal hydrides. Because they incorporate hydrogen in the +1 oxidation state, these compounds can act equally a source of the H+ ion in water.

Metal hydrides, on the other manus, contain hydrogen leap to a metal. Because these compounds contain hydrogen in a -1 oxidation state, they dissociate in h2o to give the H- (or hydride) ion.
![]()
The H- ion, with its pair of valence electrons, can abstract an H+ ion from a water molecule.
![]()
Since removing H+ ions from water molecules is one way to increase the OH- ion concentration in a solution, metal hydrides are bases.
![]()
A like blueprint can be institute in the chemical science of the oxides formed by metals and nonmetals. Nonmetal oxides dissolve in h2o to form acids. COtwo dissolves in water to give carbonic acrid, And thenthree gives sulfuric acid, and P4O10 reacts with water to give phosphoric acid.

Metallic oxides, on the other mitt, are bases. Metallic oxides formally comprise the O2- ion, which reacts with water to give a pair of OH- ions.
![]()
Metal oxides therefore fit the operational definition of a base of operations.
![]()
We see the aforementioned pattern in the chemistry of compounds that contain the ![]() OH, or hydroxide, group. Metal hydroxides, such every bit LiOH, NaOH, KOH, and Ca(OH)2, are bases.
OH, or hydroxide, group. Metal hydroxides, such every bit LiOH, NaOH, KOH, and Ca(OH)2, are bases.
![]()
Nonmetal hydroxides, such as hypochlorous acrid (HOCl), are acids.
![]()
The tabular array beneath summarizes the trends observed in these three categories of compounds. Metal hydrides, metal oxides, and metallic hydroxides are bases. Nonmetal hydrides, nonmetal oxides, and nonmetal hydroxides are acids.
Typical Acids and Bases
| Acids | Bases |
| Non-metal Hydrides HF, HCl, HBr, HCN, HSCN, H2S | Metal Hydrides Hi, LiH, NaH, KH, MgH2, CaH2 |
| Non-metal Oxides CO2, Thenii, Then3, NO2, P4O10 | Metal Oxides Li2O, Na2O, Yard2O, MgO, CaO |
| Not-metallic Hydroxides HOCl, HONO2, OtwoDue south(OH)2, OP(OH)three | Metal Hydroxides LiOH, NaOH, KOH, Ca(OH)2, Ba(OH)2 |
The acidic hydrogen atoms in the non-metal hydroxides in the table above aren't jump to the nitrogen, sulfur, or phosphorus atoms. In each of these compounds, the acidic hydrogen is attached to an oxygen atom. These compounds are therefore all examples of oxyacids.
Skeleton structures for eight oxyacids are given in the figure beneath. As a general rule, acids that contain oxygen take skeleton structures in which the acidic hydrogens are attached to oxygen atoms.
![]()
Why are Metallic Hydroxides Bases and Nonmetal Hydroxides Acids?
To understand why nonmetal hydroxides are acids and metal hydroxides are bases, nosotros take to look at the electronegativities of the atoms in these compounds. Let's start with a typical metal hydroxide: sodium hydroxide

The divergence betwixt the electronegativities of sodium and oxygen is very big (![]() EN = 2.5). As a result, the electrons in the Na
EN = 2.5). As a result, the electrons in the Na![]() O bond are not shared equally
O bond are not shared equally ![]() these electrons are drawn toward the more electronegative oxygen cantlet. NaOH therefore dissociates to give Na+ and OH- ions when it dissolves in water.
these electrons are drawn toward the more electronegative oxygen cantlet. NaOH therefore dissociates to give Na+ and OH- ions when it dissolves in water.
![]()
We go a very different pattern when nosotros apply the same process to hypochlorous acid, HOCl, a typical nonmetal hydroxide.

Here, the difference between the electronegativities of the chlorine and oxygen atoms is small (![]() EN = 0.28). Equally a result, the electrons in the Cl
EN = 0.28). Equally a result, the electrons in the Cl![]() O bail are shared more or less equally by the two atoms. The O
O bail are shared more or less equally by the two atoms. The O![]() H bond, on the other hand, is polar (
H bond, on the other hand, is polar (![]() EN = 1.24)
EN = 1.24) ![]() the electrons in this bond are drawn toward the more electronegative oxygen atom. When this molecule ionizes, the electrons in the O-H bail remain with the oxygen atom, and OCl- and H+ ions are formed.
the electrons in this bond are drawn toward the more electronegative oxygen atom. When this molecule ionizes, the electrons in the O-H bail remain with the oxygen atom, and OCl- and H+ ions are formed.
![]()
There is no abrupt change from metallic to nonmetal beyond a row or down a column of the periodic table. We should therefore expect to notice compounds that lie between the extremes of metallic and nonmetal oxides, or metal and nonmetal hydroxides. These compounds, such as Al2Oiii and Al(OH)3, are called amphoteric (literally, "either or both") because they can act as either acids or bases. Al(OH)three, for case, acts equally an acid when information technology reacts with a base.
![]()
Conversely, it acts equally a base when it reacts with an acid.
![]()
![]()
The Br nsted Definition of Acids and Bases
The Brnsted, or Brnsted-Lowry, model is based on a elementary assumption: Acids donate H + ions to another ion or molecule, which acts every bit a base of operations. The dissociation of water, for example, involves the transfer of an H+ ion from one h2o molecule to another to form H3O+ and OH- ions.
![]()
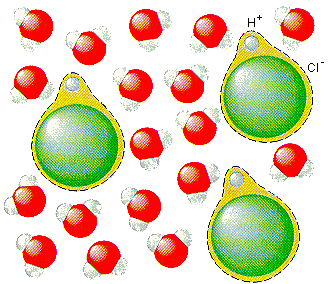
According to this model, HCl doesn't dissociate in water to form H+ and Cl+ ions. Instead, an H+ ion is transferred from HCl to a water molecule to form H3O+ and Cl- ions, equally shown in the figure beneath.
![]()

Because it is a proton, an H+ ion is several orders of magnitude smaller than the smallest atom. As a issue, the charge on an isolated H+ ion is distributed over such a small amount of infinite that this H+ ion is attracted toward any source of negative charge that exists in the solution. Thus, the instant that an H+ ion is created in an aqueous solution, information technology bonds to a water molecule. The Brnsted model, in which H+ ions are transferred from one ion or molecule to another, therefore makes more than sense than the Arrhenius theory, which assumes that H+ ions exist in aqueous solution.
Even the Brnsted model is naive. Each H+ ion that an acid donates to water is actually jump to four neighboring h2o molecules, equally shown in the figure beneath.

A more realistic formula for the substance produced when an acid loses an H+ ion is therefore H(H2O)iv +, or HnineO4 +. For all practical purposes, however, this substance can be represented equally the H3O+ ion.
The reaction between HCl and water provides the basis for understanding the definitions of a Brnsted acid and a Brnsted base. According to this theory, an H+ ion is transferred from an HCl molecule to a water molecule when HCl dissociates in water.
![]()
HCl acts as an H+-ion donor in this reaction, and HtwoO acts as an H+ ion-acceptor. A Brnsted acid is therefore any substance (such as HCl) that can donate an H+ ion to a base. A Brnsted base of operations is whatever substance (such as HiiO) that can accept an H+ ion from an acid.
There are two ways of naming the H+ ion. Some chemists call it a hydrogen ion; others call it a proton. As a event, Brnsted acids are known as either hydrogen-ion donors or proton donors. Brnsted bases are hydrogen-ion acceptors or proton acceptors.
From the perspective of the Brnsted model, reactions between acids and bases always involve the transfer of an H+ ion from a proton donor to a proton acceptor. Acids can be neutral molecules.
![]()
They tin also be positive ions
![]()
or negative ions.
![]()
The Brnsted theory therefore expands the number of potential acids. It also allows united states of america to determine which compounds are acids from their chemical formulas. Any compound that contains hydrogen with an oxidation number of +1 can be an acrid. Brnsted acids include HCl, HtwoS, H2COthree, H2PtFvi, NHfour +, HSOfour -, and HMnO4.
Brnsted bases can be identified from their Lewis structures. According to the Brnsted model, a base of operations is whatsoever ion or molecule that can accept a proton. To understand the implications of this definition, look at how the prototypical base, the OH- ion, accepts a proton.
![]()
The only fashion to take an H+ ion is to class a covalent bail to it. In order to form a covalent bond to an H+ ion that has no valence electrons, the base must provide both of the electrons needed to form the bond. Thus, only compounds that take pairs of nonbonding valence electrons tin can act as H+-ion acceptors, or Brnsted bases.
The following compounds, for instance, tin all act as Brnsted bases considering they all contain nonbonding pairs of electrons.

The Brnsted model expands the listing of potential bases to include any ion or molecule that contains i or more pairs of nonbonding valence electrons. The Brnsted definition of a base applies to so many ions and molecules that it is almost easier to count substances, such as the post-obit, that can't be Brnsted bases because they don't have pairs of nonbonding valence electrons.

![]()
The Office of H2o in the Brnsted Theory
The Brnsted theory explains water'south role in acrid-base reactions.
- Water dissociates to course ions past transferring an H+ ion from i molecule acting equally an acid to some other molecule acting as a base.
| H2O(l) | + | H2O(l) | | H3O+(aq) | + OH-(aq) |
| acid | base of operations |
- Acids react with water by altruistic an H+ ion to a neutral water molecule to form the H3O+ ion.
| HCl(chiliad) | + | H2O(l) | | H3O+(aq) | + Cl-(aq) | |
| acid | base of operations |
- Bases react with water by accepting an H+ ion from a h2o molecule to form the OH- ion.
| NH3(aq) | + | HtwoO(l) | | NHiv +(aq) | + OH-(aq) |
| base | acid |
- Water molecules tin act as intermediates in acrid-base of operations reactions by gaining H+ ions from the acrid
| HCl(g) | + | HtwoO(50) | | H3O+(aq) | + Cl-(aq) |
and and then losing these H+ ions to the base.
| NHiii(aq) | + | H3O+(aq) | | NH4 +(aq) | + HtwoO(50) |
The Brnsted model can be extended to acid-base reactions in other solvents. For example, there is a small trend in liquid ammonia for an H+ ion to be transferred from one NHthree molecule to another to grade the NHiv + and NHtwo - ions.
| 2 NH3 | | NH4 + | + NH2 - |
By analogy to the chemistry of aqueous solutions, we conclude that acids in liquid ammonia include any source of the NH4 + ion and that bases include whatsoever source of the NH2 - ion.
The Brnsted model can even be extended to reactions that don't occur in solution. A classic example of a gas-stage acrid-base reaction is encountered when open containers of full-bodied muriatic acid and aqueous ammonia are held side by side to each other. A white deject of ammonium chloride soon forms every bit the HCl gas that escapes from one solution reacts with the NHiii gas from the other.
| HCl(g) | + NH3(g) | | NH4Cl(due south) |
This reaction involves the transfer of an H+ ion from HCl to NH3 and is therefore a Brnsted acid-base reaction, even though information technology occurs in the gas stage.
![]()
What Ions Do Bases Produce,
Source: https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch11/acidbase.php
Posted by: gibsonyessund.blogspot.com


0 Response to "What Ions Do Bases Produce"
Post a Comment